Improving Clinical Outcomes with our Precision AI platform
neuropacs™ is built to meet the toughest challenges in neurodegenerative clinical research. By providing AI-powered diagnostic insights and quantitative imaging biomarkers, our platform helps CROs and pharmaceutical companies run faster, more efficient, and more successful clinical trials.

Why Trials Fail — and How We Help?
neuropacs™ isn’t just imaging, it’s a platform for precision trial success.
Costly Recruitment
Up to 50% of trial budgets are spent on patient recruitment. Screen failures are common due to diagnostic uncertainty.
Our solution:
neuropacs™ stratifies patients with unmatched accuracy, reducing screen fails and accelerating enrollment.
Unclear Endpoints
Many trials fail to meet endpoints because disease progression is difficult to measure with conventional tools.
Our solution:
Our imaging markers provide quantitative, reproducible progression metrics that detect subtle changes beyond volumetric MRI.
Inefficient Progress Monitoring
Traditional imaging and clinical assessments often miss subtle changes in disease progression. This leads to delayed signals of drug efficacy and higher risk of trial failure.
Our solution:
neuropacs™ provides ultra-sensitive imaging biomarkers that detect microstructural changes over shorter intervals, enabling earlier proof of efficacy and data-driven go/no-go decisions.
Key benefits for CROs and sponsors
Identify eligible patients faster with precision stratification and increase trial efficiency with accurate diagnostic insights.
- Accelerated recruitment
- Reduced Screen Fails
- Optimized Study Design
- Regulatory Alignment
- Lower Costs
- Higher Success Rates
Measure
Quantitative progression markers for efficacy assessment
Stratify
Advanced diagnostic tools for precise patient stratification
Optimize
Precision inputs bolster clinical endpoint achievement
Comprehensive Toolset
Delivered via secure cloud-based workflow
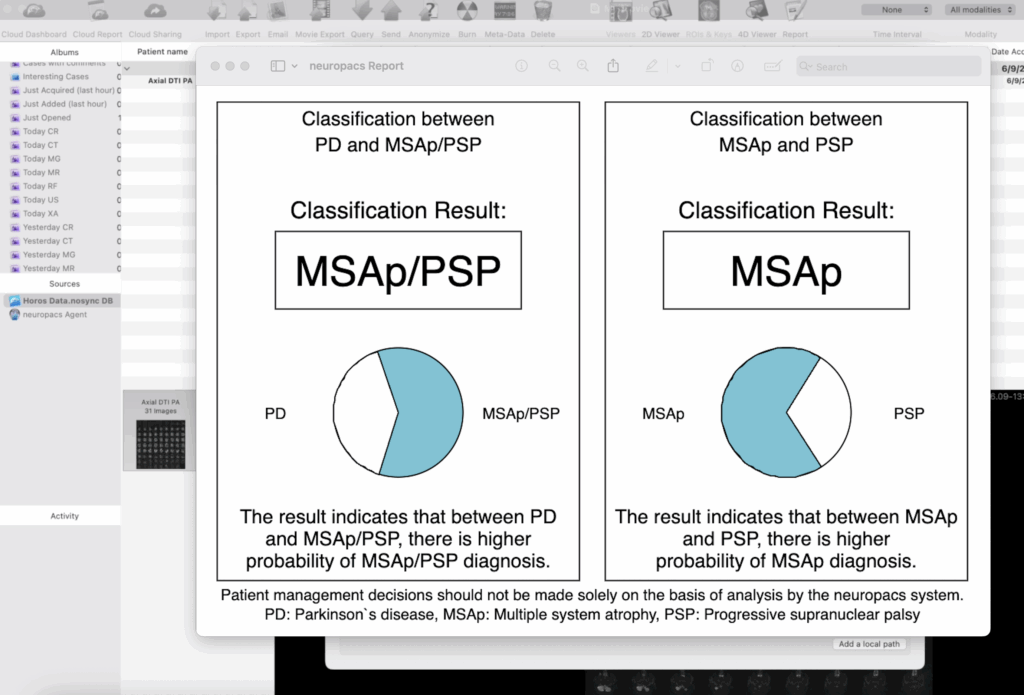
Unmatched Accuracy
Up to 96% precision in distinguishing PD from MSAp and PSP
Whether supporting clinical decision-making or powering trial success, neuropacs™ provides exclusive methodologies, actionable insights, and a seamless integration into existing imaging workflows.
96%
Up to 96% accuracy
21 sites
involved in most recent clinical trial
15+
Years of data collected
1000+
Unique datasets analyzed
Performance you can trust
neuropacs™ leverages proprietary, patent-protected techniques that combine:
-
AI-driven diagnostics trained on
harmonized multi-site data - Exclusive Patent: US 10,758,170 for Parkinsonism
- Patent pending for Dementia and Alzheimer’s
- Free-Water Imaging (FWI): A powerful diffusion MRI method that enhances early disease detection
- FDA DeNovo device clearance: anticipated in 2025. (Currently, this product is investigational and not yet cleared by the FDA).
Our Patents
Patents and patent-pending filings cover multiple neurodegenerative indications, including:
•Alzheimer’s Disease
•Parkinson’s Disease
•Multiple System Atrophy (MSA)
•Progressive Supranuclear Palsy (PSP)
•Dementia with Lewy Bodies
•Amyotrophic Lateral Sclerosis (ALS)
•Motor Neuron Disease (MND)
•Corticobasal Degeneration
•Huntington’s Disease Multiple Sclerosis (MS)
Evidence and Expertise


















neuropacs™ is the result of decades of research led by world-class clinicians and neuroscientists from:
•University of Florida
•UF Health Norman Fixel Institute for Neurological Diseases
Validated across thousands of MRI scans and multiple clinical trials, neuropacs™ delivers:
•Peer-reviewed results
•Academic rigor
•Real-world applicability
•FDA enabled
•Most sensitive platform available
Partner With Us
Whether you’re designing a Phase II study or running a global Phase III trial, neuropacs™ provides the diagnostic clarity, patient stratification, and sensitivity needed to bring neurodegenerative treatments to patients faster.
