NIH-funded clinical trial accelerates the launch of innovative software for Parkinson’s Disease diagnostic.
GAINESVILLE, FL – neuropacsTM Corp., a leading innovator in AI-driven neurological diagnostics, announces the results of a prospective multicenter study that evaluated neuropacs™ AI technology for diagnosing Parkinson’s disease and atypical parkinsonism. The study was published in JAMA Neurology (doi: 10.1001/jamaneurol.2025.0112) and funded by grant U01NS119562 from the National Institutes of Health (National Institute of Neurological Disorders and Stroke).
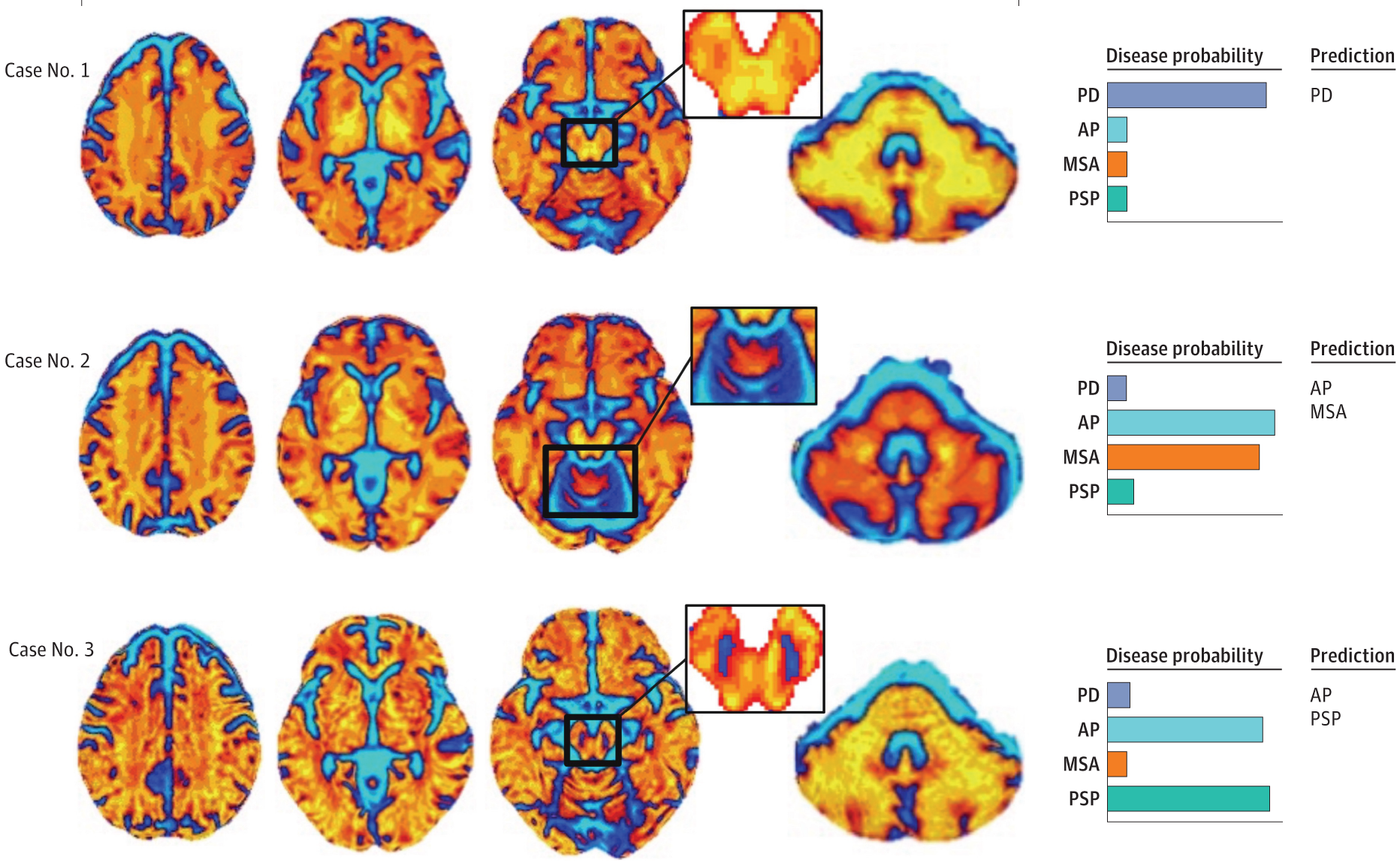
The study “Prospective Multicenter Study of Automated Imaging Differentiation for Parkinsonism” evaluated the diagnostic accuracy of neuropacs™ AI technology in 249 patients across 21 movement disorders centers of the Parkinson’s Study Group in the United States and Canada. Diffusion MRI scans were acquired in patients using Siemens, General Electric, and Philips 3T scanners. The MRI scans were used in the neuropacs™ AI software to predict the clinical diagnosis. neuropacs™ was 96% accurate for differentiating Parkinson’s disease (PD) and atypical parkinsonism, including multiple system atrophy parkinsonian variant (MSA-P) and progressive supranuclear palsy (PSP), and 98% accurate for differentiating MSA-P and PSP. Furthermore, the neuropacs™ diagnosis was confirmed in 93.9% of brains evaluated at postmortem autopsy.
The diagnosis and differentiation of PD, MSA, and PSP remain a critical challenge in the clinical environment, even for the most experienced clinicians. Up to 45% of patients clinically diagnosed with PD are later found to have another form of parkinsonism. Clinical-ready biomarkers are desperately needed to help steer clinicians toward a more specific diagnosis during a diagnostic workup. The outcome of this study positions neuropacs™ as the most accurate and validated AI technology currently available on the market for diagnosing and differentiating three common neurodegenerative forms of parkinsonism.
“This is an important milestone for the parkinsonism community, including clinical providers and, most importantly, patients”, said David Vaillancourt, Ph.D., a Professor and the Orchid Endowed Chair of the Department of Applied Physiology & Kinesiology at the University of Florida, neuropacs™ co-Founder & Chief Scientific Officer, and Principal Investigator of the study. “We anticipate that the neuropacs™ AI technology will revolutionize the current standard of care for diagnosing parkinsonism and will have far-reaching impacts on clinical care and clinical trial testing of new therapeutics”.
The Parkinson’s Study Group is the largest non-profit PD clinical trial site network in North America, with over 155 sites and 375 credentialed investigators. The Automated Imaging Differentiation for Parkinsonism study was conducted in 21 sites, including 19 in the United States and two in Canada. The internationally-renowned Fixel Institute was also one of the sites who contributed to various aspects of the study. “The findings highlight the transformative potential of this diagnostic software in clinical practice”, stated Michael Okun, M.D., the co-director of the Norman Fixel Institute for Neurological Diseases and the Medical Advisor for the Parkinson’s Foundation, as well as a UF Health neurologist and an uncompensated member of the neuropacs™ Board of Clinical Advisors. He adds “the age of using data-driven tools like AI to diagnose Parkinson’s and other neurological conditions has arrived. As clinicians, we must embrace these technologies and start considering how we can best integrate them into routine workflows for neurological care.”
Kristophe Diaz PhD, Executive Director and Chief Science Officer of CurePSP, added that “Proper diagnosis of PSP remains a critical need to decrease burden for people living with the disease and ensure they receive proper clinical care as soon as possible. The results of this study are exciting progress towards a non-invasive and clinically scalable solution to help improve diagnosis. We look forward to seeing how the findings of this study can further impact the PSP community and advance our shared goal of better outcomes for all.”
For more information about neuropacsTM and its revolutionary diagnostic solutions, visit neuropacs.com.
About neuropacsTM:
neuropacsTM is a pioneering neurological technology company dedicated to developing innovative AI-driven diagnostics. With a commitment to advancing healthcare through cutting-edge technologies, neuropacsTM remains at the forefront of revolutionizing clinical care standards.
Forward-Looking Statements
This communication includes express and implied “forward-looking statements.” Forward-looking statements include all statements that are not historical facts and in some cases, can be identified by terms such as “may,” “might,” “will,” “could,” “would,” “should,” “expect,” “intend,” “plan,” “objective,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “continue,” “ongoing,” or the negative of these terms, or other comparable terminology intended to identify statements about the future. By their nature, these statements are subject to numerous risks and uncertainties, that could cause actual results, performance or achievement to differ materially and adversely from those anticipated or implied in the statements. You should not rely upon forward-looking statements as predictions of future events. Although our management believes that the expectations reflected in our statements are reasonable, we cannot guarantee that the future results, performance, or events and circumstances described in the forward-looking statements will be achieved or occur. Recipients are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date such statements are made and should not be construed as statements of fact. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, any future presentations, or otherwise, except as required by applicable law.